
Basic Characteristics
This FDA-approved Class II medical device (K233077) was developed under my leadership alongside a multinational team of engineers and medical advisors.
I worked directly with patients and technical staff, employing Human-Centered Design principles to align the product with medical and user requirements while addressing market needs.
From concept to stable production, the device was completed within two years and is certified by ANVISA under code 80488299002. It remains in the market, with thousands of units sold over the years by a single small manufacturer.
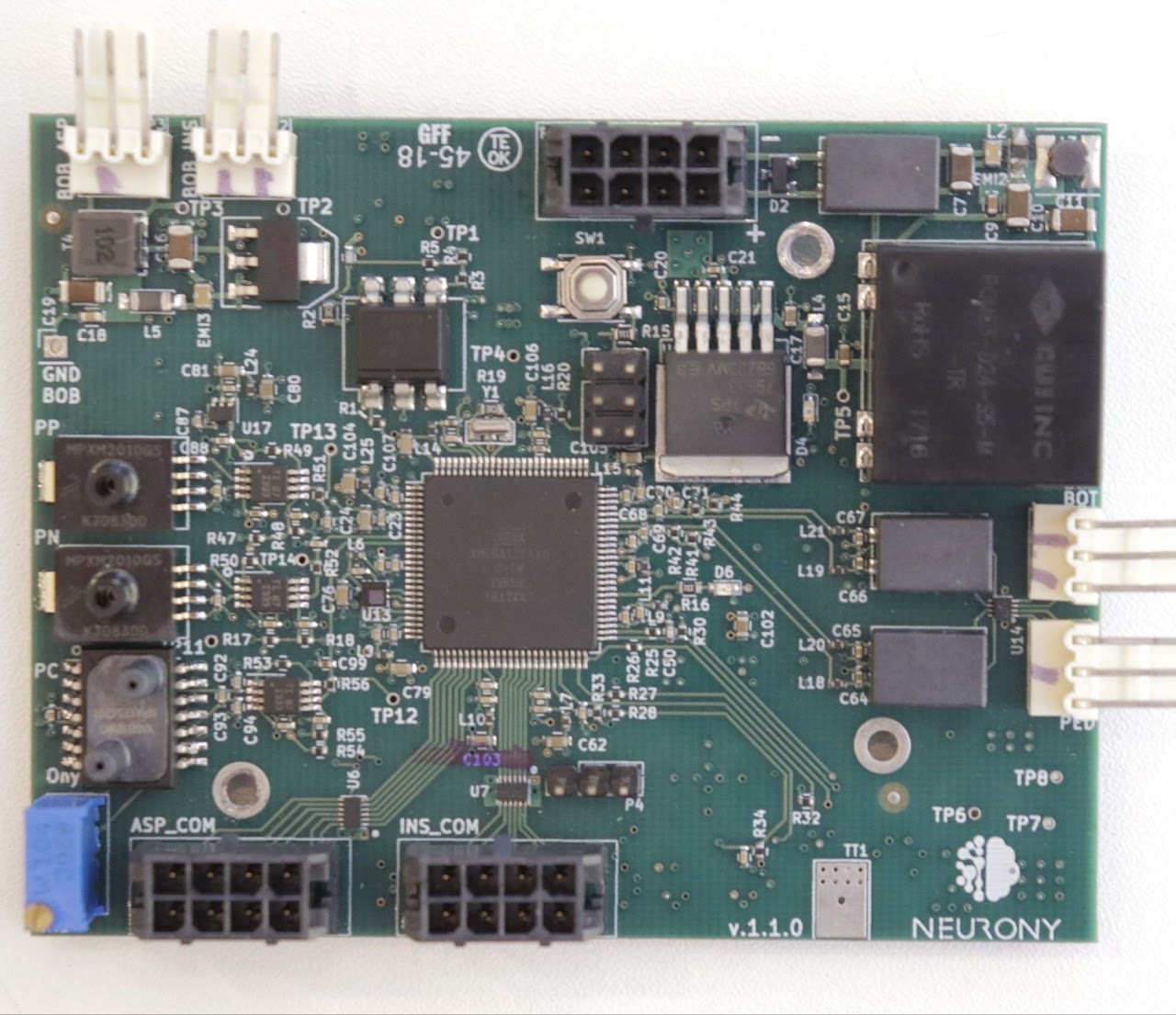
System Characteristics
- Compliant with IEC 60601-1, 60601-1-2, 60601-1-6, 60601-1-9, 60601-1-11.
- Firmware designed with a hard real-time premise.
- Controls two 150W BLDC motors with fast startup and DC braking.
- Integrated pressure and flow conversion.
- 7" touchscreen interface controlled by a single 8-bit microcontroller extracting every possible FLOP of the microcontroller.
- Lower electronic cost compared to competitors.
- Three four-layer PCBs that taught me a lot about common-mode EMC noise.

Areas that I had specifically worked on or led
- Product Owner.
- Developed main embedded code in C.
- Designed the BLDC motor controller board.
- Represented the company during certification testing.
- Key member of the Software Documentation team and Risk Management Committee.
- Designed and documented production and testing equipment.
- Performed comparative performance analysis against competitors.
Gallery
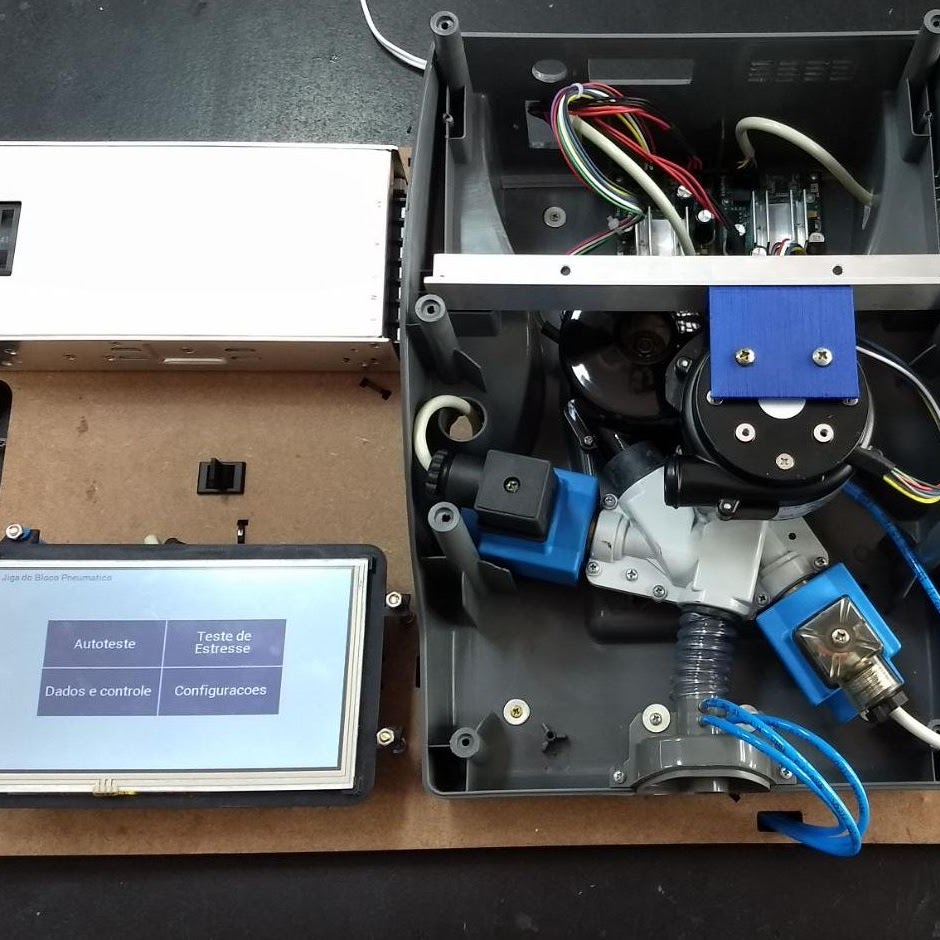
One of the production test jigs

IEC 60601-1 vibration tests

IEC 60601-1-2 radiated emissions test
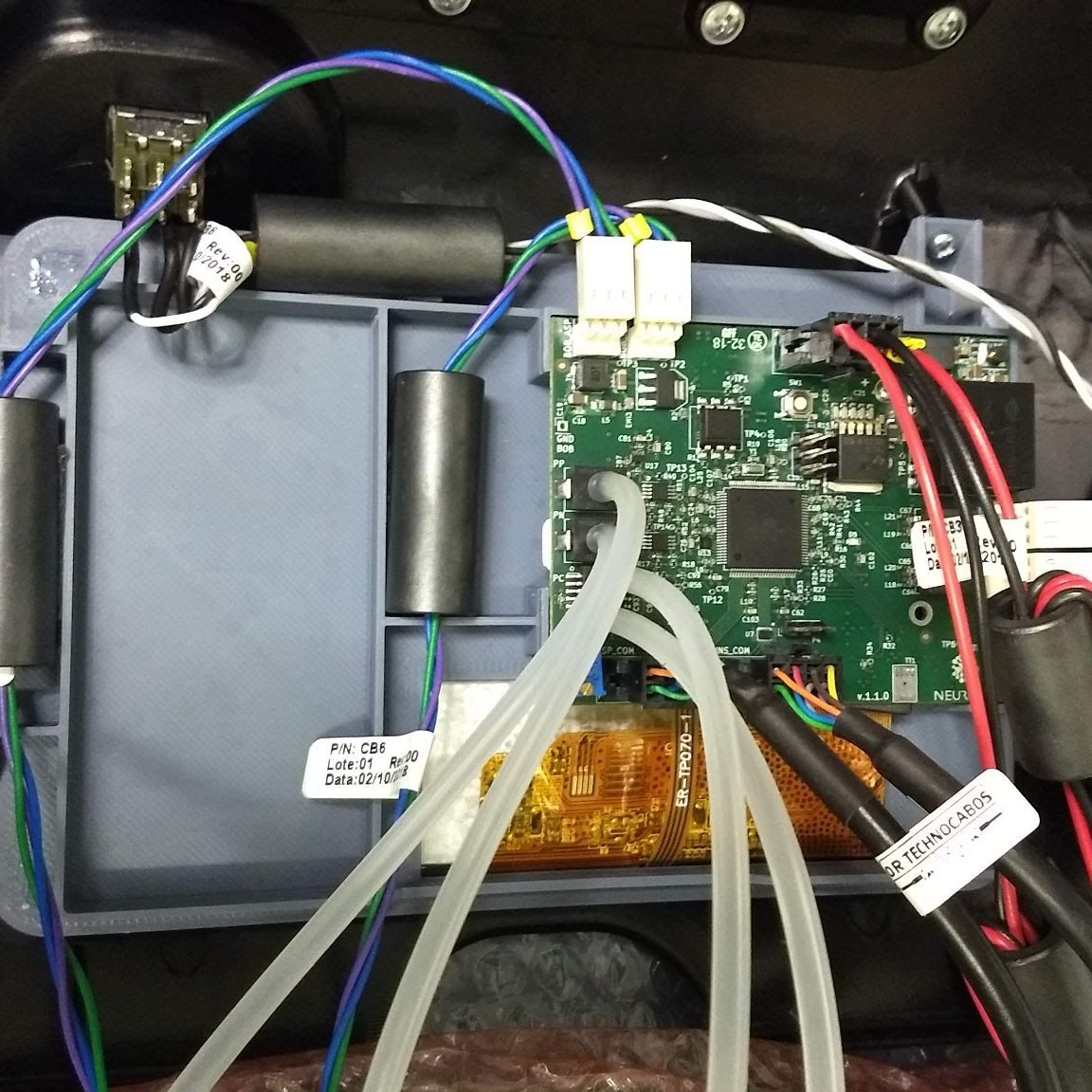
Many ferrites as the result of a poor EMC design
